When you find yourself ready, an auditor will perform a “Stage two assessment” to determine If the Business meets the conventional’s necessities.
Delivering a design to adhere to when establishing and functioning a administration program, learn more about how MSS do the job and where they may be applied.
Referred to as a “Phase a single assessment”, our auditor will perform an First assessment of one's administration program to find out In the event the Main demands in the standard are now being met. We are going to offer an in depth report outlining the locations you might want to target so as to comply with the requirements.
The class includes a exam at the end to confirm knowledge and competence, and it's only having an accredited program that a person may become authorised to audit to get a certification entire body.
In this way, you'll be able to pinpoint nonconformities and their root results in within the get-go and style and design important actions to mitigate and deal with them.
ISO 14001 Schooling CoursesDiscover the significance of environmental management with Qualified schooling designed to satisfy business expectations.
Once the overview, the management finalizes an index of action merchandise located in internal audits and enhancements being implemented. The list can then be employed for a guiding document for the following management critique before applying for your certification.
All ISO criteria are reviewed each individual 5 years to ascertain whether a revision is required to maintain them present-day and relevant to the Market.
Accredited classes for individuals and pros who want the highest-good quality training and certification.
Coach your critical men and women about ISO 27001 needs and provide cybersecurity consciousness instruction to all your employees.
ISO 13485 would be the internationally identified typical for high-quality administration techniques in the look and manufacture of medical gadgets. It outlines precise requirements that support businesses ensure their health-related products fulfill both consumer and regulatory needs for safety and efficacy.
All ISO standards are reviewed each individual five years to determine if a revision is needed in order to preserve it current and suitable for your Market. ISO 13485:2016 is developed to answer the latest good quality administration program tactics, which includes changes in know-how and regulatory necessities and expectations.
Interior audit – The internal audit is in position that you should Verify your QMS processes. The intention is to make certain documents are in place to confirm compliance on the procedures and to find troubles and weaknesses that may or else remain hidden.
So as to satisfy field ideal methods and regulatory prerequisites, ISO 13485 Certification this set of ISO benchmarks incorporate specific standards that a corporation needs to reveal compliance with.
 Amanda Bearse Then & Now!
Amanda Bearse Then & Now!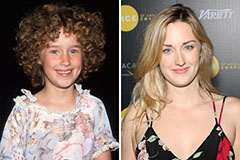 Ashley Johnson Then & Now!
Ashley Johnson Then & Now! Katie Holmes Then & Now!
Katie Holmes Then & Now! Keshia Knight Pulliam Then & Now!
Keshia Knight Pulliam Then & Now!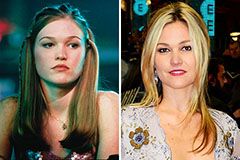 Julia Stiles Then & Now!
Julia Stiles Then & Now!